Abstract
Background: Chemorefractory primary and secondary central nervous system lymphomas (CNSL) have a dismal prognosis, and represent a major unmet need in oncology. To address this, we conducted a pilot trial of axi-cel in patients with relapsed/refractory CNSL. All patients had Ommaya reservoirs, such that after CAR-T infusion, paired CSF and blood could be collected and analyzed daily, allowing the interrogation of single-cell CAR-T transcriptional profiles at an unprecedented level of detail. This analysis enabled us to address key mechanistic questions relevant to CAR-T therapy for CNS lymphoma, including determining the impact of tumor site (brain vs lymphoid tissue) on CAR-T efficacy and toxicity, and discovering the drivers of CAR-T cell transcriptional evolution as these cells move between their sites of action.
Methods: Adult patients with either primary or secondary relapsed/refractory CNSL were enrolled on the 'Axi-cel In CNS Lymphoma' Trial (Clinicaltrials.gov# NCT04608487). Patients received fludarabine/cyclophosphamide lymphodepletion followed by axi-cel infusion. Blood and CSF were sampled daily between D0-14 after infusion, with data from CAR-T maximum expansion (Days 5-9) reported here. 5' 10x Single-cell RNA-Seq (scRNA-Seq) and TCR-Seq were performed on peripheral blood mononuclear cells (PBMC), enriched T cells, and CSF-derived cells. scRNA-Seq data was analyzed with the Python packages 'scanpy' and 'scVI' to identify and characterize high-quality CD4+ and CD8+ T cells, and to conduct differential expression tests. Gene set enrichment analysis (GSEA) was used to compare CSF and blood CD4+ and CD8+ CAR-T cells using the 'clusterProfiler' package in R.
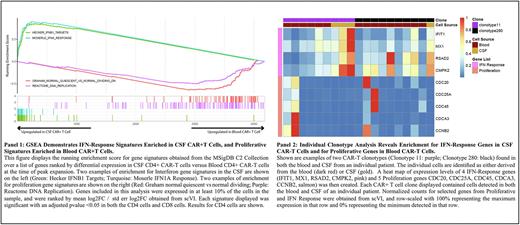
Results: Detailed clinical results from this trial are reported in an accompanying abstract. In brief, data from the first 7 patients on-study demonstrated no treatment-limiting toxicities and an ORR of 86%, with 6 of 6 responding patients achieving a CR by 3 months. 2 of the responding patients have progressed, 1 at 6 months and 1 at 15 months. To interrogate the mechanisms driving axi-cel efficacy in inducing CNS responses, we analyzed 129,088 CAR+ and non-CAR T cells from the peripheral blood (64,337 cells), the CSF (37,070 cells), and the axi-cel product (27,681 cells) from 5 enrolled patients, with blood/CSF collected around the time of maximum CAR-T expansion (Days 5-9 post-infusion). Enumeration of CAR-T cells in the blood vs CSF revealed a mean 13% CAR-Ts of total blood T cells (range 7-24%), and a mean 18% (range 13-28%) in the CSF. CD4+ CAR-T cells predominated in the infused product (mean 79% CAR-T cells were CD4+, range 68-95%), with the CD4:CD8 balance maintained at peak expansion (with 77% (range 60-83%) CAR-T cells in the blood being CD4+, and 95% (range 91-97%) CAR-T cells in the CSF being CD4+). Sc-TCR sequencing enabled an evaluation of the T cell repertoire, revealing a similarly broad CAR-T repertoire in both the blood (mean 1,375 clones (range 313-2,563) and CSF (mean 1,090 clones, range 268-1,965). scRNA-Seq gene expression and clustering analysis revealed a striking distinction between blood and CSF CAR-Ts: While blood CAR-Ts exhibited a prominent proliferation gene expression signature (analyzed by single cell GSEA), CSF CAR-Ts, obtained on the same days as those from the blood, exhibited strong enrichment for interferon-pathway associated genes (Panel 1). Although the majority of CAR-T clones identified were unique to either the blood or CSF, we were able to identify 54 shared clones between these compartments. Analysis of these clones further substantiated the transcriptional evolution of CAR-Ts between the blood and CSF. Thus, individual shared clones tracked in blood and CSF from multiple patients exhibited an evolution from a more diverse proliferation/IFN pathway profile in the blood, to predominantly IFN pathways enriched in CSF CAR-Ts (Panel 2).
Conclusions: Daily examination of the blood and CSF by scRNA-Seq and scTCR-Seq has identified a major distinction between blood and CSF CAR-Ts at the level of individual CAR-T clones, revealing the evolution of a prominent interferon pathway transcriptomic signature of CAR-Ts in the CSF. These results suggest that IFN pathway enrichment may play a major role in CAR-T efficacy for the eradication of CNS disease in patients with CNSL.
Disclosures
Gerdemann:AlloVir: Divested equity in a private or publicly-traded company in the past 24 months, Patents & Royalties. Poddar:UCLA: Other: intellectual Property, Patents & Royalties; Kite, a Gilead Company: Current Employment, Current equity holder in publicly-traded company. Mattie:Kite/Gilead: Current Employment. Jacobson:Precision BioSciences: Consultancy, Honoraria, Other: Travel Support; Ispen: Consultancy, Honoraria; Lonza: Consultancy, Honoraria, Other: Travel Support; Nkarta: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria; Humanigen: Consultancy, Honoraria, Other: Travel Support; Axis: Speakers Bureau; ImmPACT Bio: Consultancy, Honoraria; Daiichi Sankyo: Consultancy, Honoraria; Kite, a Gilead Company: Consultancy, Honoraria, Research Funding; Celgene: Other: Travel Support; bluebird bio: Consultancy, Honoraria; Instil Bio: Consultancy, Honoraria; Epizyme: Consultancy, Honoraria; Clinical Care Options: Speakers Bureau; Pfizer: Other: Travel Support, Research Funding; Novartis: Consultancy, Honoraria, Other: Travel Support; BMS/Celgene: Consultancy, Honoraria. Kean:HiFiBio: Consultancy; Mammoth Biosciences: Current equity holder in private company; Bristol Myers Squibb: Research Funding; Vertex: Consultancy; EMD-Serono: Research Funding; Magenta: Research Funding; Kite/Gilead: Research Funding; Bluebird Bio: Research Funding.
OffLabel Disclosure:
Axi-cel for CNS Lymphoma
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal